Research
Our group research interest lies in the area of development of new methodologies for organic transformations, asymmetric organocatalysis, chiral catalyst design, target-oriented synthesis (TOS) of biologically important natural compounds, and diversity-oriented synthesis (DOS) of natural product-like molecules. Methodologies developed and molecules thus built are employed in the investigation of biologically significant processes. Our current research areas are described below.

New Reaction Development
Catalysis facilitated by simple organic molecules (vs. transition metal catalyzed reactions) has recently garnered much attention from many research laboratories in academia and in industry. Our contribution to this exciting field of organocatalysis is the development of organic phosphine catalysis. The phosphine catalyses that we developed are operationally simple: they only require a catalytic amount of a phosphine as a reagent. Therefore, after the reaction, simple evaporation of the reaction solvent and flash column chromatography of the crude reaction mixture provides the desired products in pure forms in typically excellent yields. We have developed over two dozen new phosphine-catalyzed reactions to date, some examples of which are illustrated below.

Chem. Sci. 2018, DOI: 10.1039/C7SC04381C. [pdf]
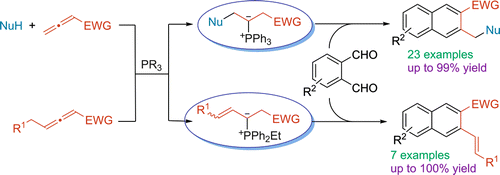
J. Am. Chem. Soc. 2015, 137, 11258–11261. [pdf]
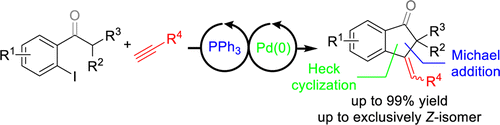
Org. Lett.. 2015, 17, 2058–2061. [pdf]
Dealkenylative Functionalizations of Alkene C(sp3)–C(sp2) Bonds
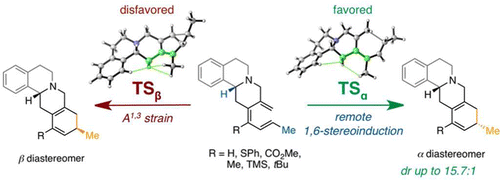
A recent focus in our laboratory is the activation of alkene C(sp3)–C(sp2) bonds commonly found in abundant plant-based terpene and terpene-derived natural products. These efforts have been shown to have applications in total synthesis and the rapid generation of biologically relevant molecules. Future efforts include developing novel variations of this methodology, as well as designing sustainable catalytic systems for these types of transformations.

J. Am. Chem. Soc. 2022, 144, 14828–14837. [pdf]

Science 2019, 364, 681–685. [pdf]
1024_1.jpg)
Science, 2023, 381, 877-886. [pdf]
Novel Catalyst Design
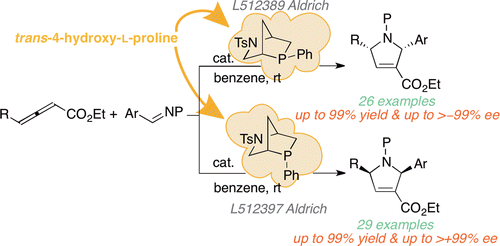
Our group developed a new family of L-hydroxyproline (Hyp)-derived [2.2.1] bicyclic phosphine catalysts, currently available from Sigma-Aldrich. These catalysts have shown great synthetic utility, as demonstrated in multiple publications (1, 2), and proven the catalytic potential of the rigid [2.2.1] bicyclic framework. We are currently exploring the use of other natural products as a starting point to develop new families of chiral phosphines that are easily tunable and can be used to facilitate a variety of asymmetric processes.

J. Am. Chem. Soc. 2022, 144, 14828–14837. [pdf]
Target-Oriented Synthesis (TOS) of Natural Products
One of the central themes of research in our laboratories is development of phosphine-catalyzed allenoate annulations and their applications in the chemical synthesis of natural products and their unnatural analogs of medicinal significance. The synthetic targets we choose are highly relevant to human health, specifically the treatment of the common cold, hypertension, amoebic dysentery, malaria, arrhythmia, and cancer. Presented below are some applications of our methodology towards the synthesis of biologically significant natural products
Diversity-Oriented Synthesis (DOS) and Chemical Biological Applications
Another area of our group research entails diversity-oriented combinatorial synthesis (DOS) of libraries of small organic molecules and their applications in chemical genetics. In the chemical genetics approach small molecules are systematically screened for the desired biological effects. Thus, identified small molecules are used to modulate the cellular functions of their target proteins, thereby elucidating the molecular mechanism of biologically important processes. When the protein of interest is a cause for a human disease, the hit from the screening in biological assays may also find its use in therapeutic remedies. Our collaboration with biologists in our chemical genetic research resulted in the discovery of several enzyme inhibitors, on one of which we have filed a patent for the development of anti-cancer therapeutics. More recently, another small molecule, dubbed "efsevin," has been show to be a potent modulator of cardiac rhythmicity through regulation of mitochondrial Ca2+ uptake via the voltage-dependent anion channel 2 (VDAC2). It also displays prophylactic activity against ventricular tachycardia in a rodent model of a human heart disease.
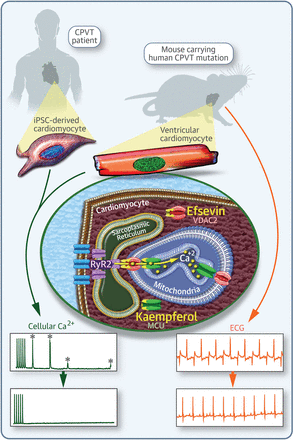

eLife 2015, 4, e04801. [pdf]
JACC: Basic Translational Science. 2017, 2, 727-737. [pdf]